Half-Life Calculator-with solutions
Blog advanced calculator, Basic Calculator, calculator scientific calculator, desmos calculator scientific, desmos science, desmos scientific calculator, exponential notation calculator, fraction calculator, Half-Life Calculator, sci notation calculator, scientific notation calculatorFor students learn about radioactive decay mean lifetime and the definition and formula derivation of the relationship between half life constants and the Half-Life Calculator is a crucial tool in physics and chemistry.
Half-Life Calculator
Calculate radioactive decay with precision and visualize half-life progression
Nuclear Decay Principles
Half-life is the time required for half of the radioactive atoms in a sample to decay. This exponential decay follows the equation: N = N₀ × (1/2)^(t/T), where N is the remaining amount, N₀ is initial amount, t is time elapsed, and T is half-life.
Decay Parameters
Isotope Presets
(5730 years)
(30.17 years)
(3.8 days)
Results
Remaining Quantity
Decay Progress
Nuclear Physics Calculator | For educational purposes | Shows exponential decay characteristics
Half-Life Calculator
To determine how long it takes for a drug to decompose to half of its initial amount and a half-life calculator is frequently employed. It becomes crucial in nuclear physics when examining unstable isotopes. Additionally it is used in environmental investigations as well as chemistry and pharmacology. It is possible to make extremely accurate forecasts regarding the decay process by using a half-life calculator. This guarantees accuracy in intricate computations while simultaneously saving time.
Also because it defines the stability of elements and the idea of half-life is essential. Scientists and students can use this calculator to quickly determine findings and prevent human errors.

A black handheld scientific half-life calculator displaying isotope 60 with a time of 8 hours, a decay constant of 0.0231 per hour, and a calculated half-life of 30 hours on the screen.
Define Formula
Half-life (t₁/₂): The time required for the concentration of a reactant to decrease to half of its initial concentration.
Rate constant (k): A proportionality constant in the rate law that relates the rate of reaction to the concentration of reactants.
The relationship between half-life and rate constant depends on the order of the reaction.
🧮 Derivation of Formulae
First-order reaction
Rate law:
$$
\frac{d[A]}{dt} = -k[A]
$$
Integrating:
$$
[A] = [A]_0 e^{-kt}
$$
At half-life, $[A] = \frac{[A]_0}{2}$:
$$
\frac{[A]0}{2} = [A]_0 e^{-k t{1/2}}
$$
Dividing by $[A]_0$:
$$
\frac{1}{2} = e^{-k t_{1/2}}
$$
Taking natural log:
$$
\ln \left(\frac{1}{2}\right) = -k t_{1/2}
$$
$$
t_{1/2} = \frac{\ln 2}{k}
$$
✅ For first-order, half-life is constant and independent of concentration.
Second-order reaction
Rate law:
$$
\frac{d[A]}{dt} = -k[A]^2
$$
Integrated form:
$$
\frac{1}{[A]} = \frac{1}{[A]_0} + kt
$$
At half-life, $[A] = \frac{[A]_0}{2}$:
$$
\frac{1}{[A]0/2} = \frac{1}{[A]_0} + k t{1/2}
$$
$$
\frac{2}{[A]0} = \frac{1}{[A]_0} + k t{1/2}
$$
$$
t_{1/2} = \frac{1}{k [A]_0}
$$
✅ For second-order, half-life depends on the initial concentration.
Zero-order reaction
Rate law:
$$
\frac{d[A]}{dt} = -k
$$
Integrated form:
$$
[A] = [A]_0 – kt
$$
At half-life, $[A] = \frac{[A]_0}{2}$:
$$
\frac{[A]0}{2} = [A]_0 – k t{1/2}
$$
$$
k t_{1/2} = \frac{[A]_0}{2}
$$
$$
t_{1/2} = \frac{[A]_0}{2k}
$$
✅ For zero-order, half-life decreases as concentration decreases.
Half Life
The time it takes for a radioactive material to decay to 50% of its initial value is known as its half-life. For instance just half of the initial atoms will be left in a substance with a half-life of ten years. Only a fraction is left after another ten years.
How long a drug is active in the body is determined by its half-life in medicine. Studying the characteristics of unstable isotopes is beneficial in physics. Therefore the half life provides a comprehensive grasp of the processes of decline both natural and man-made.
Mean Lifetime
Another important concept in research on radioactive decay is the mean lifetime. The mean lifetime is the average amount of time until a nucleus decays while half-life concentrates on the period for 50% reduction. Although the mean lifetime is mathematically longer than the half-life there is a constant factor that connects the two.
The mean duration offers a more profound insight into atom stability in radioactive processes. Despite their differences both numbers are necessary for correctly assessing decay rates. It is possible to express decay forecasts in terms of probability by knowing the mean lifetime.
Definition and Formula Derivation of the Relationship Between Half Life Constants
Accuracy in science depends on the definition and formula derivation of the link between half life constants. The mathematical relationship between the decay constant (λ) and half-life (T½) is as follows:
T2 = ln(2) / λ
The decline constant denoted by λ is the likelihood of a nucleus decaying per unit of time. The relationship between half-life and mean duration is also demonstrated by the definition and formula derivation of the relationship between half-life constants. The average lifespan is determined by:
τ = 1 / λ
Together these result in the following conclusion:
τ = T½ / ln(2)
Decline probability or mean lifespan and half-life are thus properly connected by the definition and formula derivation of the link between half-life constants.
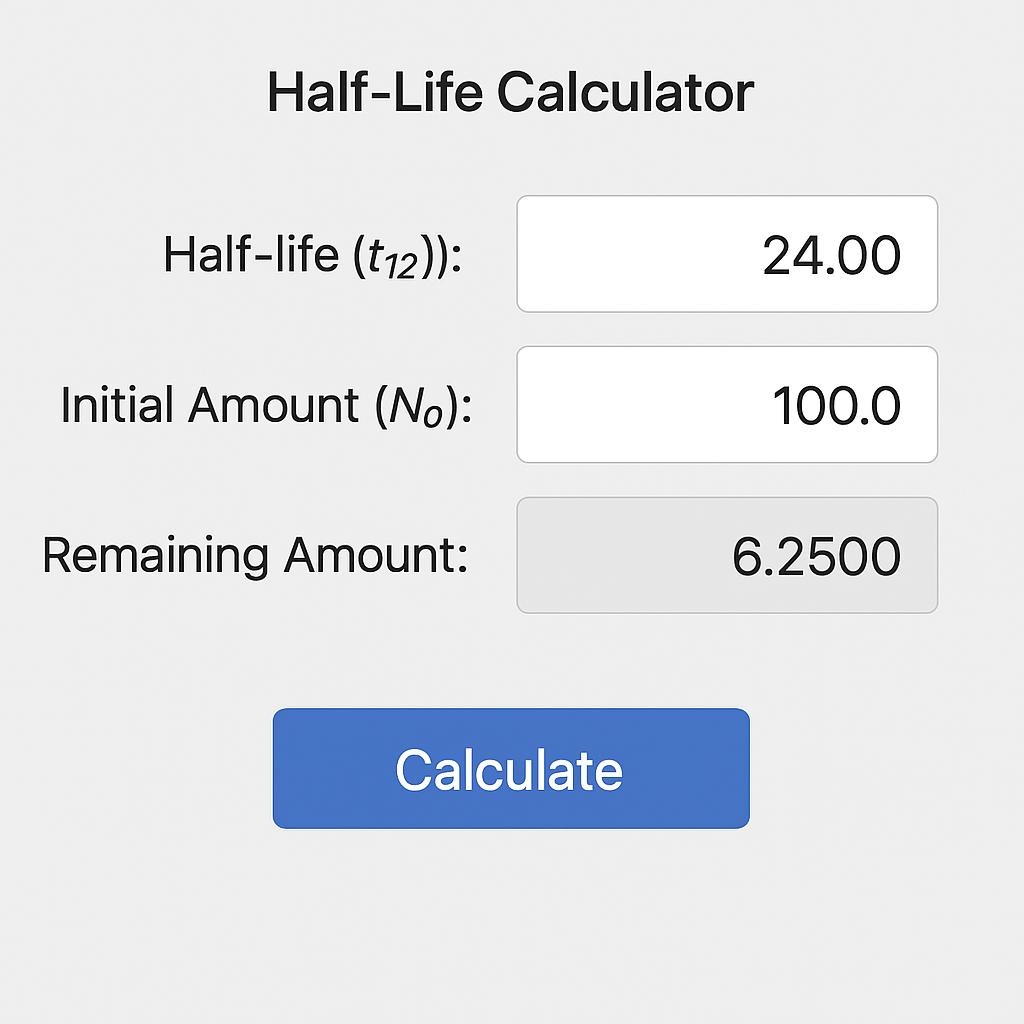
“Minimalist digital Half-Life Calculator interface showing half-life input of 24, an initial amount of 100, and a calculated remaining amount of 6.25 with a blue calculate button.
Applications of Half-Life Calculator
There are real-world uses for the Half-Life Calculator outside of nuclear physics. It aids medical professionals in understanding the duration of pharmacological activity. It helps determine the age of remains in archaeology by using radiocarbon dating. The Half-Life Calculator is a tool used by environmentalists to forecast how long pollutants will persist in the environment.
The Half-Life Calculator also becomes a universal scientific instrument because it may be used in a variety of domains. As a result it gives experts and students alike a trustworthy way to calculate.
Conclusion
In conclusion a comprehensive understanding of radioactive decay may be formed by combining the Half-Life Calculator or half life or mean lifetime and the definition and formula derivation of the link between half life constants. These principles are vital for learning or teaching and implementing knowledge in real-world circumstances.
Therefore if that you are a professional or researcher or student use the Half-Life Calculator right now to make your complicated decay calculations easier with a few clicks!
For more Calculators follow: www.calczon.com